Hydrogen enables the long-term storage of large amounts of electrical energy, and could thus make a major contribution to the conversion of our electricity supply to renewable resources. One method of producing hydrogen is water electrolysis. The basic process is very simple: water is broken down into hydrogen and oxygen in an electrolyser using electricity. In the process, electrical energy is converted into chemical energy and stored. This process can ultimately be reversed to generate electrical energy (power supply). There are currently two systems in use for this process: alkaline electrolysers and proton exchange membrane electrolysers. However, both techniques have disadvantages. One delivers a low current density and hydrogen quality, while the other requires a lot of material and is therefore expensive.
Young technology with potential: better performance and stability
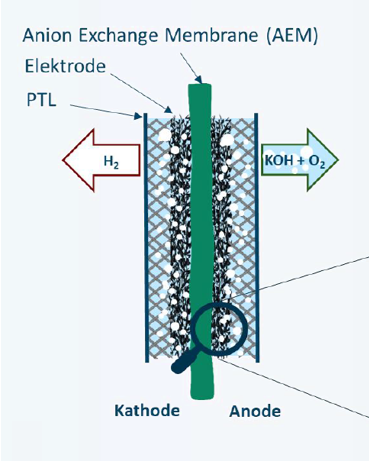
The project team is about to change this by optimization of anion exchange membrane water electrolysis (AEM-WE), a comparatively young technology that combines the advantages of both conventional technologies. The AEM Neo project aims to take a decisive step towards higher performance and longevity. The focus here is on optimizing the membrane electrode assembly (MEA) - the heart of water electrolysis cells. For conventional technologies, there are already mature membranes and ionomers or binders. These are the ion-conducting components which enable the separation of hydrogen and oxygen in the cell. However, these binders in particular are not ideal for the use in AEM-WE. Thus, in this project new binder materials are developed and suitable manufacturing processes for the entire AEM-WE cell are investigated. The necessary infrastructure for the chemical modification and characterization of the binder materials is available in the laboratories of the Institute of Chemistry of Polymeric Materials. At the same time, state-of-the-art testing facilities for testing the newly developed electrolysis cells are available at HyCentA.
The material of choice, for the first tests, is commercially available polytetrafluoroethylene (PTFE). PTFE is a universally applicable polymer, with high resistance against acids, bases, alcohols, and many other chemicals. However, as PTFE is chemically inert, it is not intrisically ion-conducting, and therefore not suitable as a binder in the AEM-WE membrane. To change this, PTFE is chemically modified at the Department of Chemistry so that protonizable groups are attached to the surface, thus providing the desired ion conductivity. In addition, the wettability is increased and the adhesion of gas bubbles is reduced as well. This is achieved by the use of various plasma-based methods, and subsequent wet-chemical treatment. Finally, the modified binder will be used to produce an AEM-WE cell, and the influence of various parameters, such as the ratio between binder and catalyst, on cell performance will be investigated.
Project name: AEM Neo - Novel Efficient Organic binder materials for AEM water electrolysis
Funding details: Future Fund of the Province of Styria (NEXT GREEN TECH)
Project partner:HyCentA Research GmbH, Institute of Chemistry of Polymeric Materials at Montanuniversität Leoben
Contact:
Dipl.-Ing. Dr.mont. Christine Bandl
christine.bandl(at)unileoben.ac.at
+43 3842 402 - 2306
Univ.-Prof. Dr. Wolfgang Kern
wolfgang.kern(at)unileoben.ac.at
+43 3842 402 - 2350